Apligraf® clinical trials.
Only Apligraf has conducted randomized controlled trials (RCTs) resulting in FDA approval for the healing of VLUs and DFUs.1-3
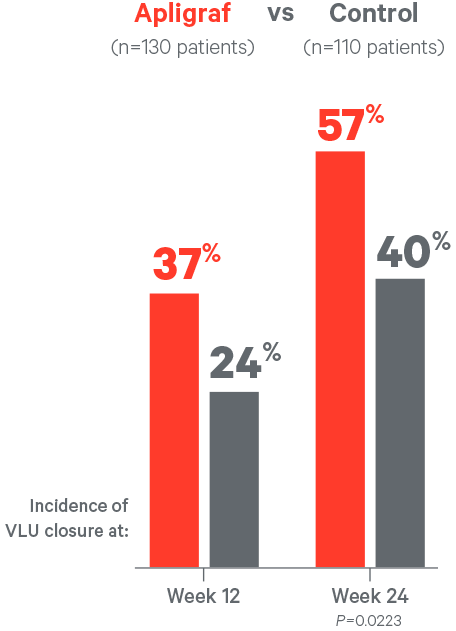
- 57% of VLUs closed by week 24 for Apligraf vs 40% for the control (P=0.0223)
- Median time to wound closure was 99 days for Apligraf vs 184 days for the control (P=0.0073)
- Apligraf closed wounds 85 days faster
- 56% of DFUs closed by week 12 for Apligraf vs 38% for the control (P=0.0042)
- Median time to wound closure was 65 days for Apligraf vs 90 days for the control (P=0.0026)
- Apligraf closed wounds 25 days faster
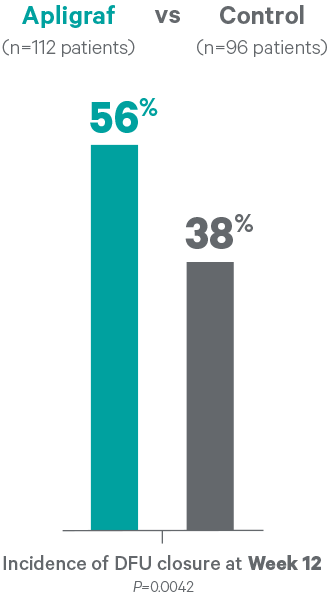
A prospective, randomized, controlled, multicenter unmasked study was conducted to evaluate the safety and efficacy of Apligraf in comparison to control treatment, saline moistened gauze, in the treatment of diabetic neuropathic foot ulcers. Wound closure was defined as 100% epithelialization without drainage.
- Osteomyelitis incidence at 6 months was 2.7% vs 10.4% for the control (P=0.04)
- Amputation (on study limb) incidence at 6 months was 6.3% vs 15.6% for the control (P=0.028)
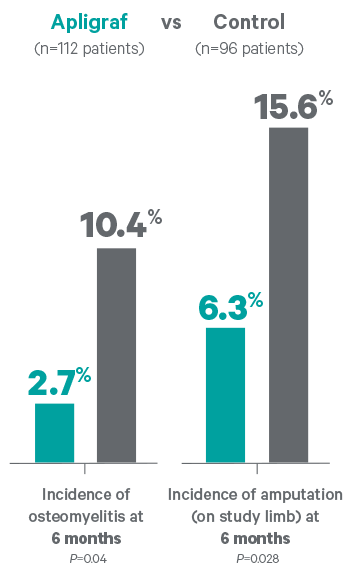
This study was not designed to determine the incidence of osteomyelitis or amputations. These data were acquired through evaluation of clinical trial adverse events.
Stimulating wound closures in real-world studies
See the effectiveness of Apligraf in real-world studies, or contact an Organogenesis Tissue Regeneration Specialist to see how Apligraf can help your practice.
Contact usPlease refer to the Apligraf Package Insert for complete prescribing information.
REFERENCES:
- Apligraf [package insert]. Canton, MA: Organogenesis Inc; 2017.
- Data on file. Organogenesis Inc.
- Veves A, et al. Diabetes Care. 2001;24(2):290-295.