Apligraf® commercial insurance coverage support.
When it comes to treating VLUs and DFUs, 100% of commercial medical policies cover Apligraf.*1 The commercial payer summary guidelines below show what the policies are for a number of insurance providers.
Commercial insurance coverage
100%
of commercial medical policies cover Apligraf treatment for VLUs and DFUs*1
Commercial payer summary guidelines
Aetna Wound Care Policy #0244
Effective: 05/28/1998
Last Review: 07/29/2022
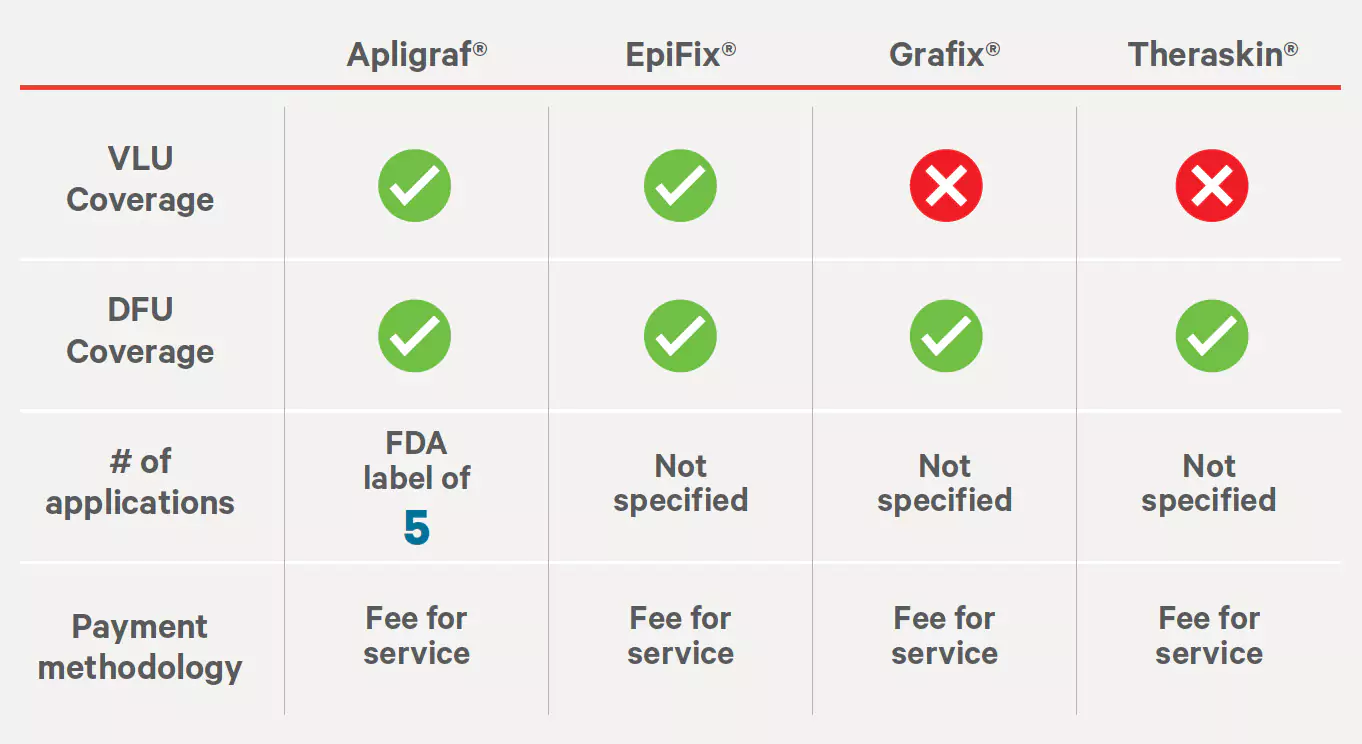 Source: http://www.aetna.com/cpb/medical/data/200_299/0244.html
Source: http://www.aetna.com/cpb/medical/data/200_299/0244.htmlAnthem Medical Coverage Policy #SURG 00011
Allogeneic, Xenographic, Synthetic and Composite Products for Wound Healing and Soft Tissue Grafting
Effective: 06/29/2022
Last Review: 05/12/2022
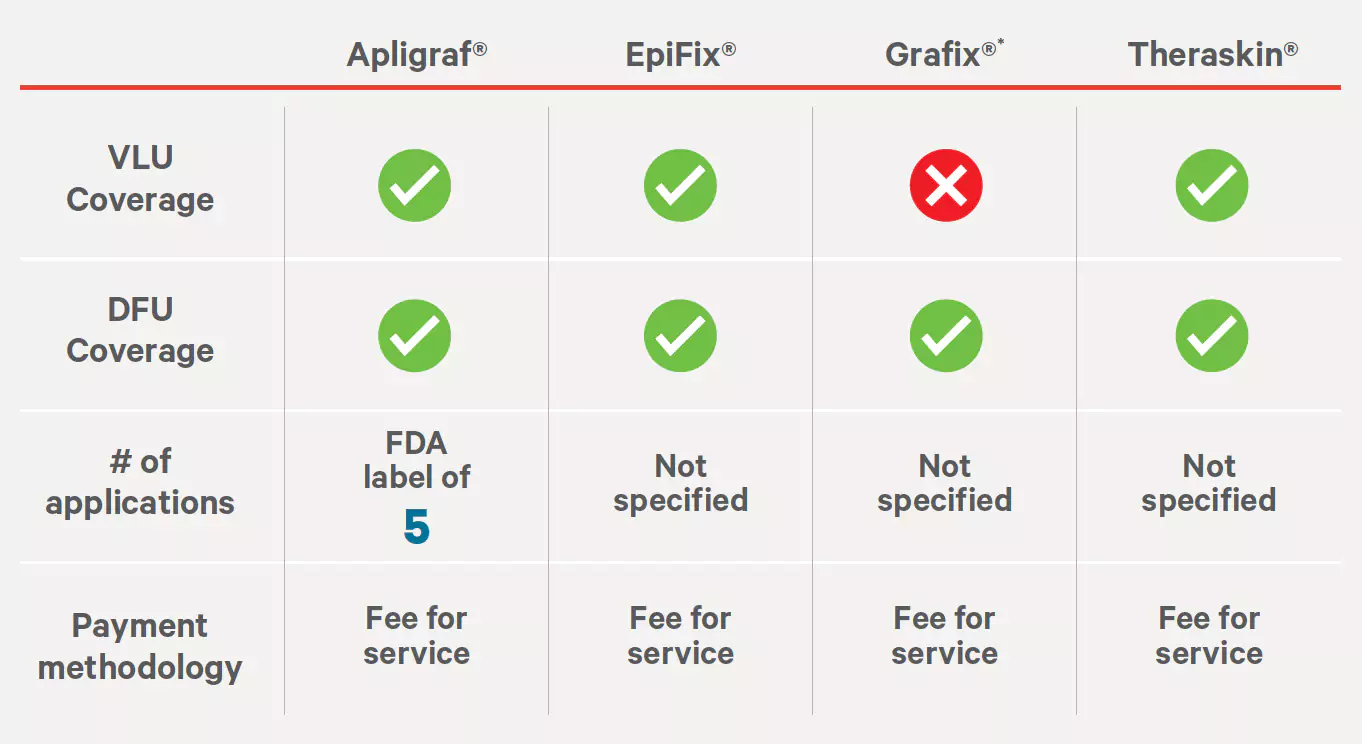 Source: https://www.anthem.com/dam/medpolicies/abcbs/active/policies/mp_pw_a053309.html †Only Grafix Prime is covered for DFUs.
Source: https://www.anthem.com/dam/medpolicies/abcbs/active/policies/mp_pw_a053309.html †Only Grafix Prime is covered for DFUs.BC/BS Federal Employee Program
Bioengineered Skin and Soft Tissue Substitutes Policy #FEP7.01.113
Effective 04/01/2022
Amniotic Membrane and Amniotic Fluid Policy #FEP 7.01.149
Effective: 07/01/2022
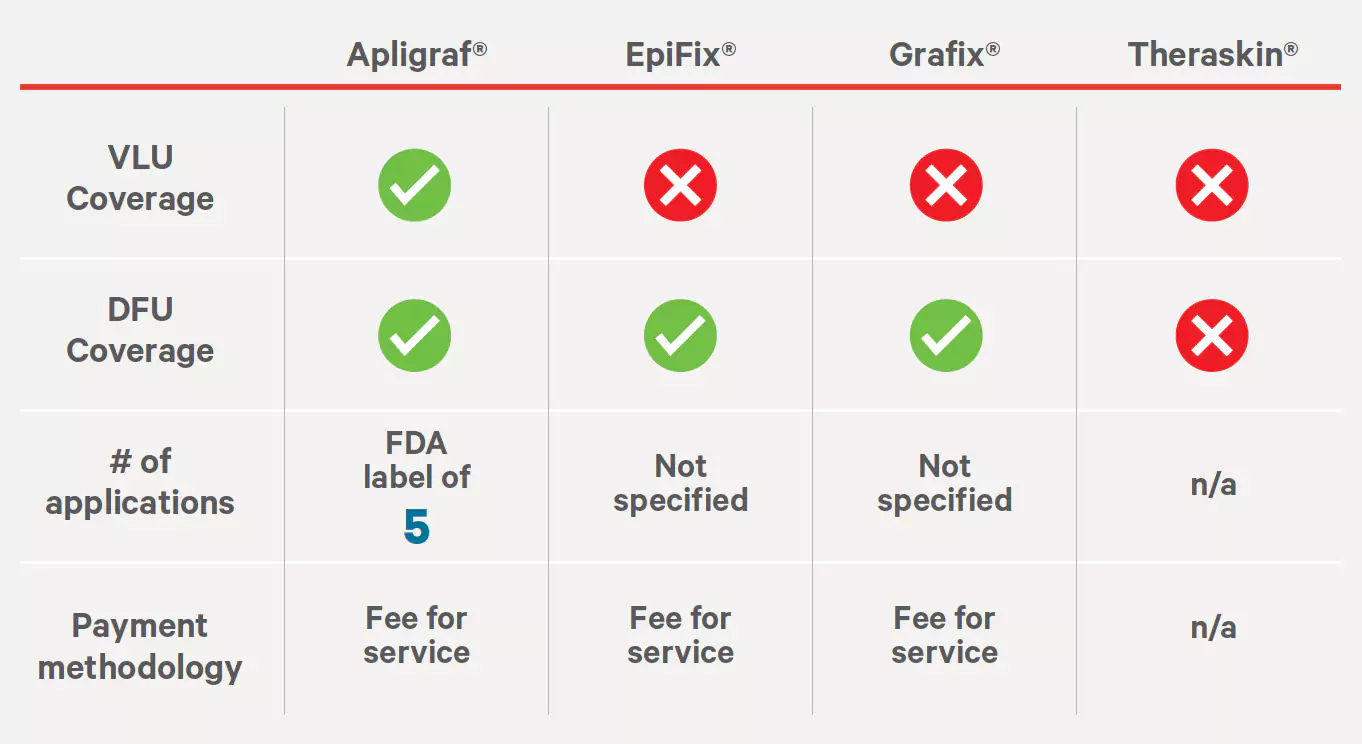 Sources: https://www.fepblue.org/-/media/PDFs/Medical%20Policies/3-18-2022/Medical_Policies/701113%20Bioengineered%20Skin%20and%20Soft%20Tissue.pdf https://www.fepblue.org/-/media/PDFs/Medical%20Policies/07-06-2022/701149%20Amniotic%20Membrane%20and%20Amniotic.pdf
Sources: https://www.fepblue.org/-/media/PDFs/Medical%20Policies/3-18-2022/Medical_Policies/701113%20Bioengineered%20Skin%20and%20Soft%20Tissue.pdf https://www.fepblue.org/-/media/PDFs/Medical%20Policies/07-06-2022/701149%20Amniotic%20Membrane%20and%20Amniotic.pdfBC/BS Highmark Commercial Medical Policy—Pennsylvania #S-33-035 & #S-249-007
Effective: 01/01/2021
Last Review: 12/21/2020
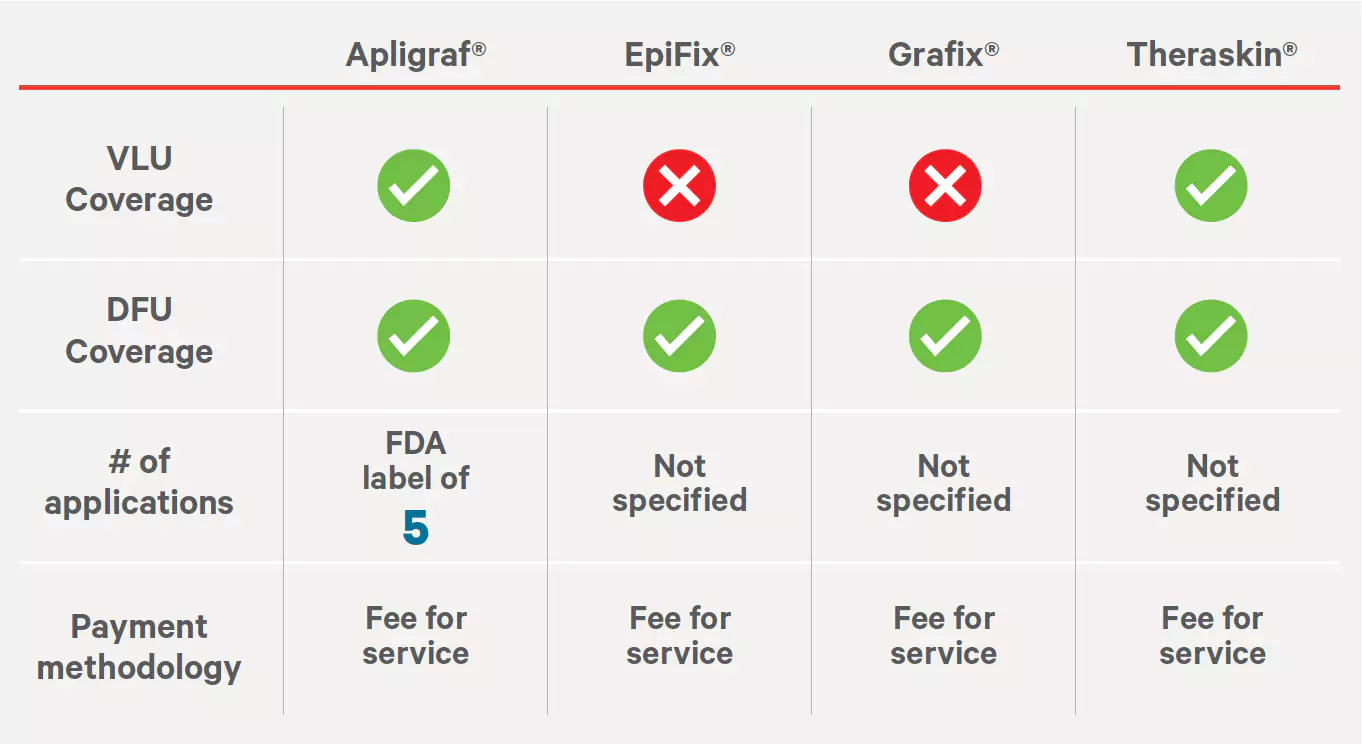 Sources: https://securecms.highmark.com/content/medpolicy/en/highmark/pa/commercial/policies/Surgery/S-33/S-33-035.html https://securecms.highmark.com/content/medpolicy/en/highmark/pa/commercial/policies/Surgery/S-249/S-249-007.html
Sources: https://securecms.highmark.com/content/medpolicy/en/highmark/pa/commercial/policies/Surgery/S-33/S-33-035.html https://securecms.highmark.com/content/medpolicy/en/highmark/pa/commercial/policies/Surgery/S-249/S-249-007.htmlCigna Medical Policy #0068
Tissue-Engineered Skin Substitutes
Effective: 05/15/2022
Next Review: 03/15/2023
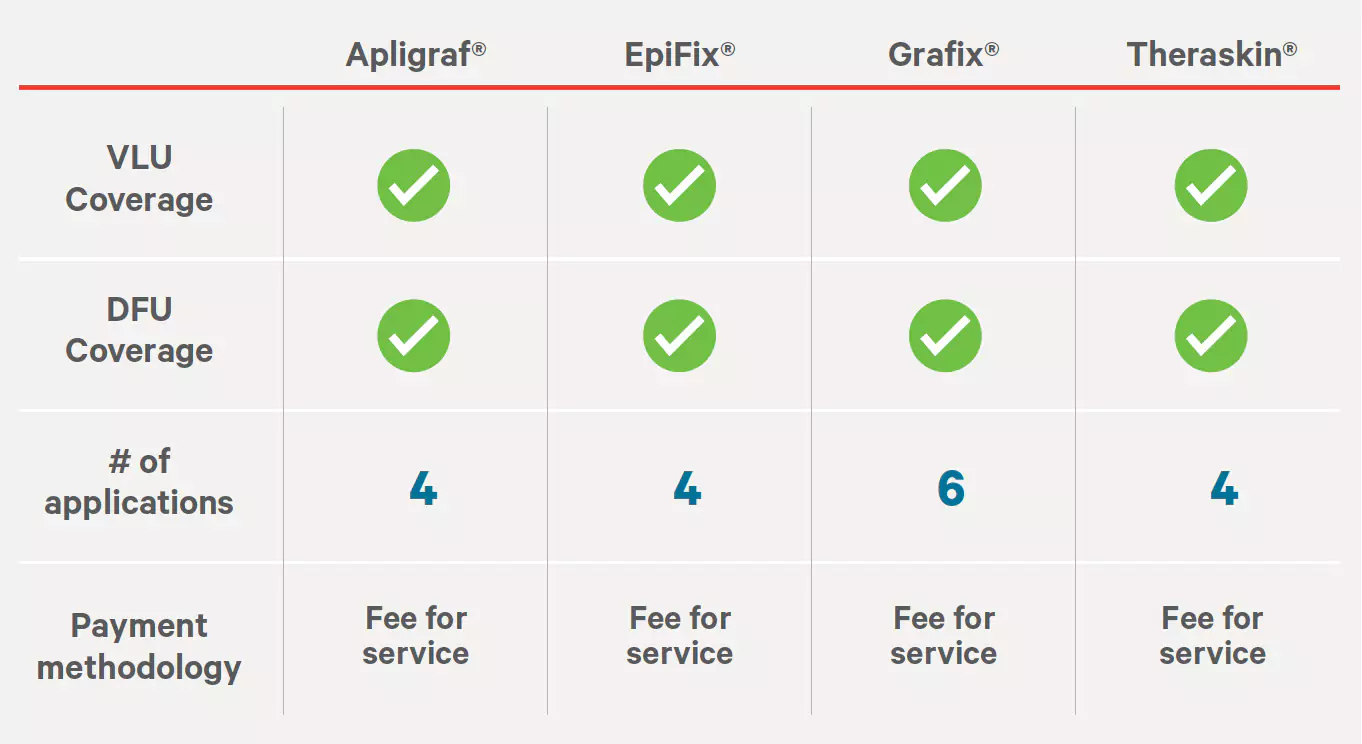 Source: https://cignaforhcp.cigna.com/public/content/pdf/coveragePolicies/medical/mm_0068_coveragepositioncriteria_woundhealing.pdf
Source: https://cignaforhcp.cigna.com/public/content/pdf/coveragePolicies/medical/mm_0068_coveragepositioncriteria_woundhealing.pdfExcellus Medical Coverage Policy #7.01.35
Bioengineered Tissue Products for Wound Treatment and Surgical Interventions
Effective: 04/15/2021
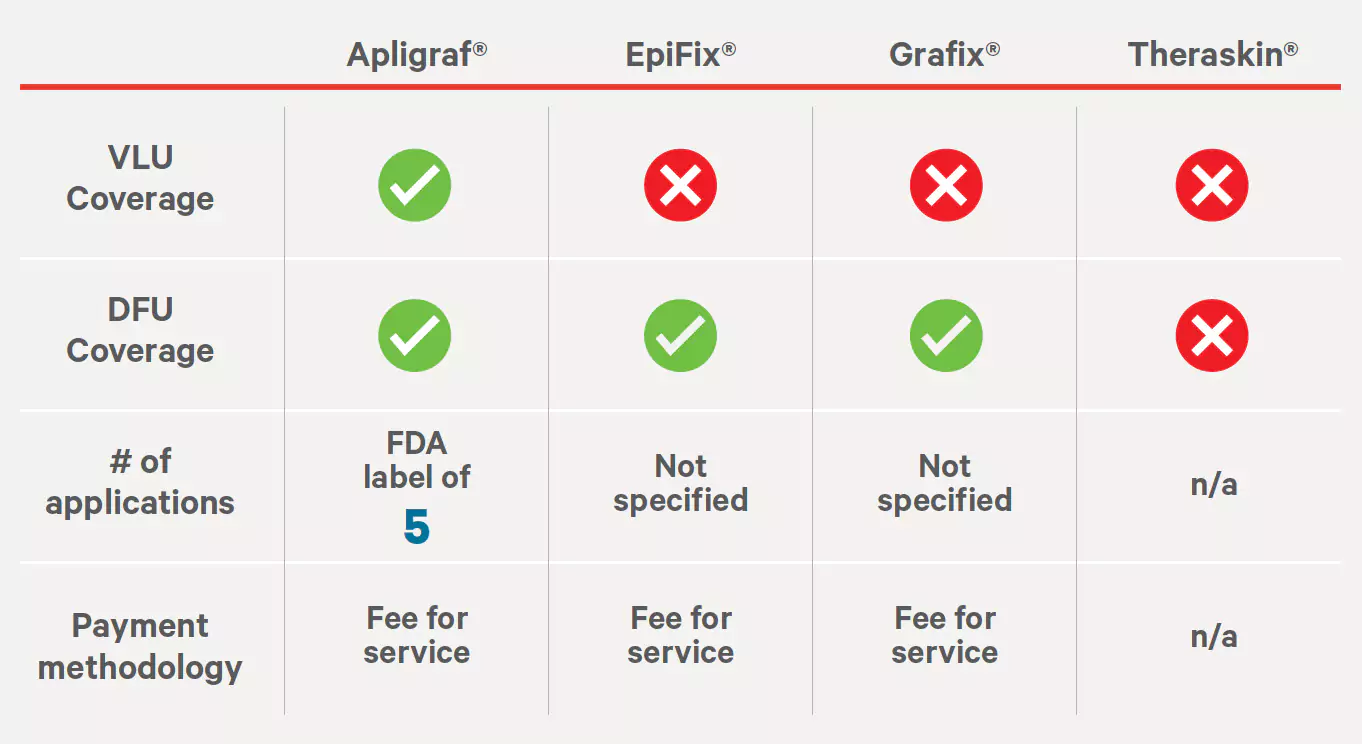 Source: https://provider.excellusbcbs.com/documents/20152/127121/bioengineered-tissue-products.pdf/ae9812e6-205c-a3a3-4377-c1e367687672?t=1563463233746
Source: https://provider.excellusbcbs.com/documents/20152/127121/bioengineered-tissue-products.pdf/ae9812e6-205c-a3a3-4377-c1e367687672?t=1563463233746Humana Medical Policy #HCS-0370-039
Skin and Tissue Substitutes
Effective: 06/23/2022
Last Review: 01/27/2022
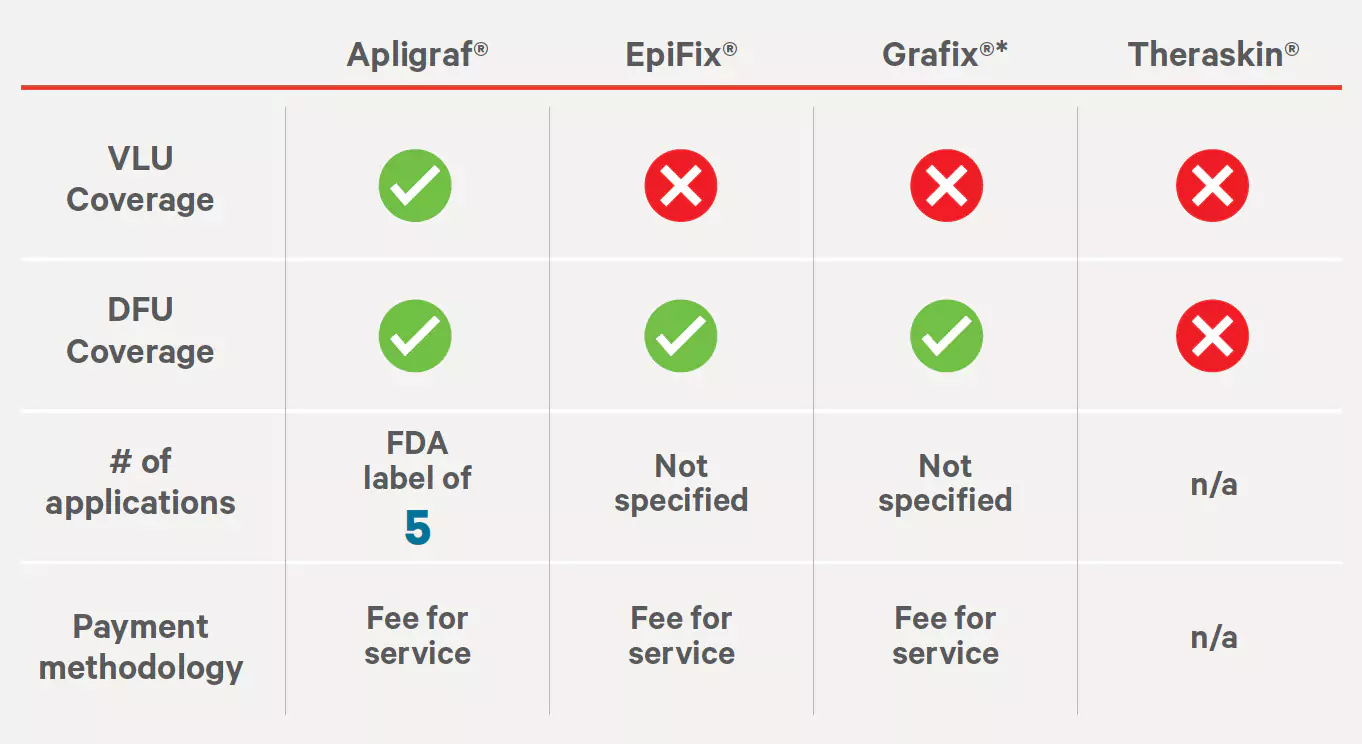 Source: https://dctm.humana.com/Mentor/Web/v.aspx?objectID=0900092982efa7d9&searchID=79c54fc6-5303-4a02-9b7f-23facd9744fe&dl=1 ‡Grafix Core and Grafix Prime.
Source: https://dctm.humana.com/Mentor/Web/v.aspx?objectID=0900092982efa7d9&searchID=79c54fc6-5303-4a02-9b7f-23facd9744fe&dl=1 ‡Grafix Core and Grafix Prime.Medica Tissue-Engineered Skin Substitutes for Wound and Surgical Care
Effective: 02/16/2022
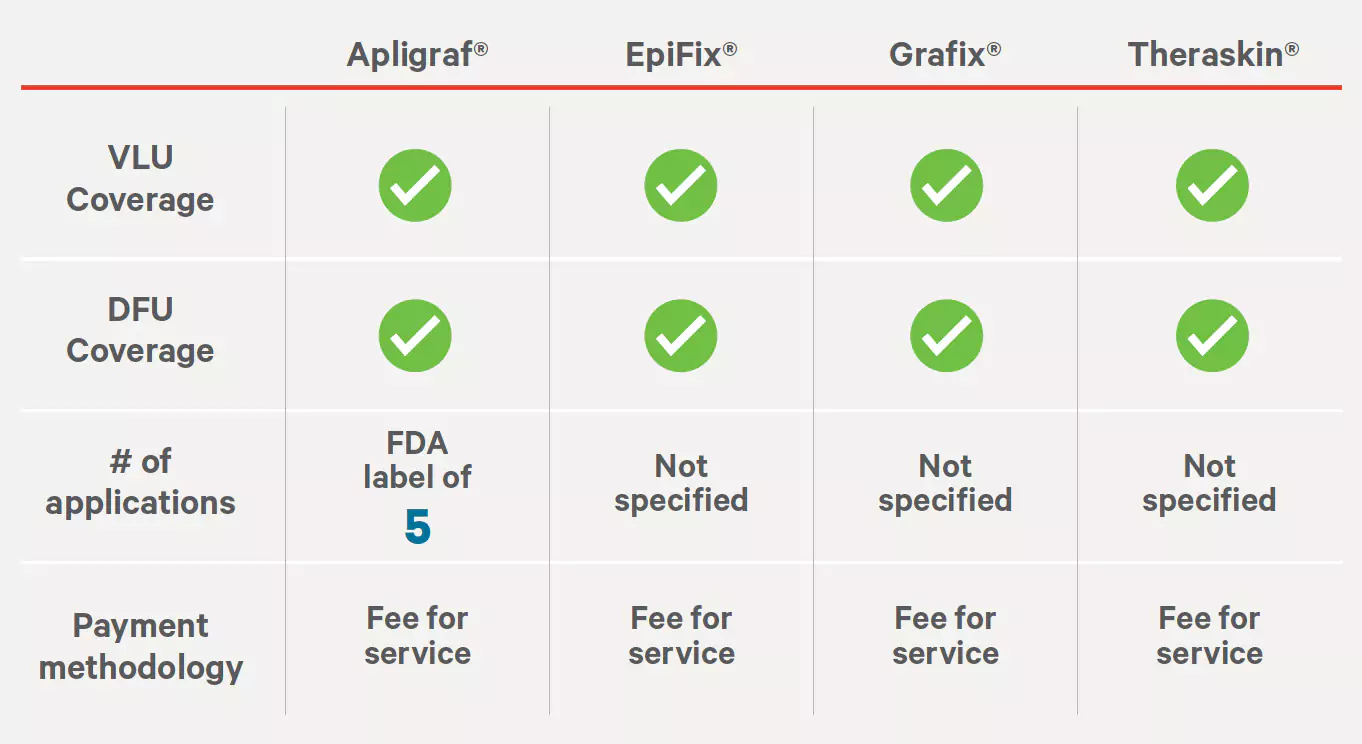 Source: https://partner.medica.com/-/media/documents/provider/coverage-policies/tissue-engineered-skin-substitutes-cp.pdf?la=en
Source: https://partner.medica.com/-/media/documents/provider/coverage-policies/tissue-engineered-skin-substitutes-cp.pdf?la=enUnited Health Care Commercial Medical Policy #2020T0592H
Effective: 07/01/2022
 Source: https://www.uhcprovider.com/content/dam/provider/docs/public/policies/comm-medical-drug/skin-soft-tissue-substitutes.pdf
Source: https://www.uhcprovider.com/content/dam/provider/docs/public/policies/comm-medical-drug/skin-soft-tissue-substitutes.pdfReady to help your VLU and DFU patients?
Talk to an Organogenesis Tissue Regeneration Specialist about using Apligraf, the gold standard to heal VLUs and DFUs faster.
Contact usPlease refer to the Apligraf Package Insert for complete prescribing information.
REFERENCE:
- Data on file. Organogenesis Inc.