Apligraf® product details.
See how to apply Apligraf to VLUs and DFUs. Also, view additional resources.
How to apply Apligraf
Diabetic foot ulcer application
Venous leg ulcer application
Application steps1,2
Preparing the wound bed
Apply Apligraf to a clean, noninfected, and debrided wound after irrigating the wound with a noncytotoxic solution. Ensure hemostasis following debridement.
Step 1: Preparing Apligraf
Before opening, check the package’s expiration date and pH to ensure both are within range
Apligraf is packaged with the epidermal (matte) side facing up and the dermal (glossy) side facing down
- The dermal side rests on a polycarbonate membrane; be sure to not inadvertently remove it with Apligraf
Open the pack and remove Apligraf using aseptic techniques
- Gently remove by lifting from the edge
- Apligraf must be used within 15 minutes of opening
Prior to application, Apligraf may be placed on a saline-soaked gauze and/or fenestrated to allow for drainage
 1A
1A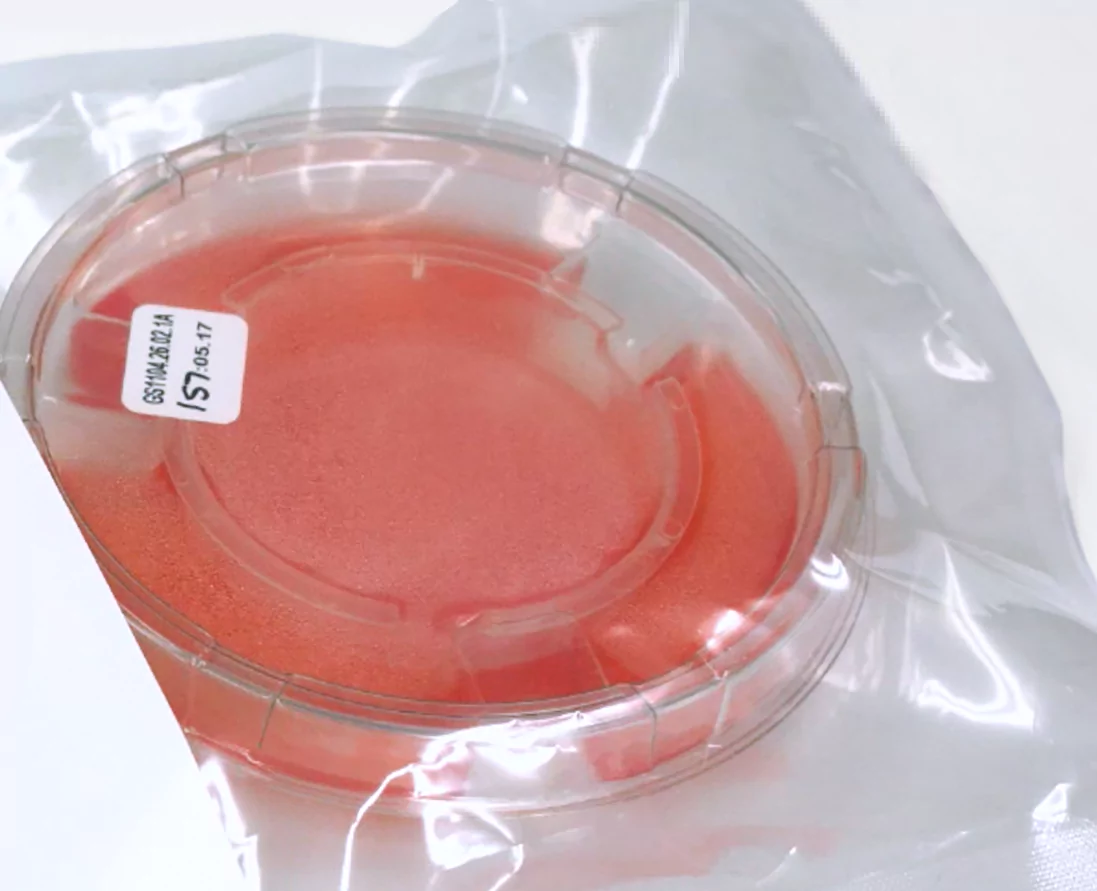 1B
1B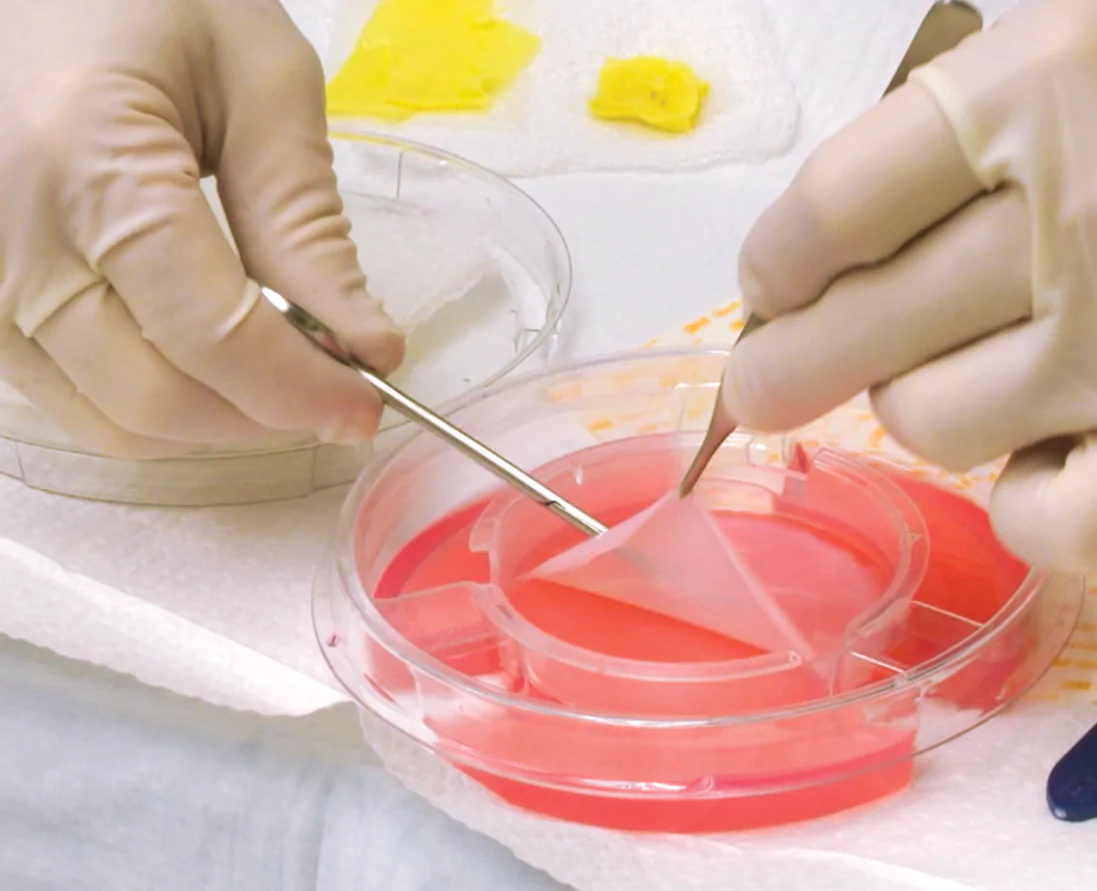 1C
1C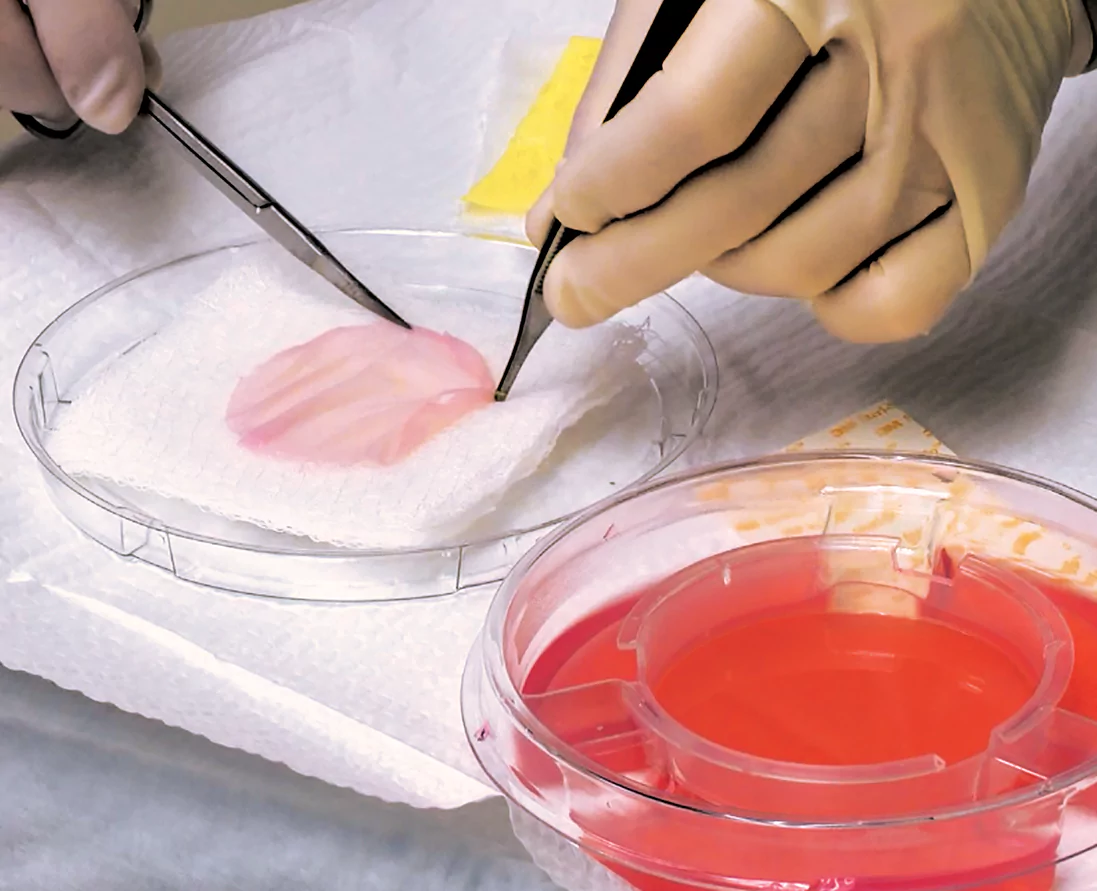 1D
1DStep 2: Applying Apligraf
Place Apligraf over the wound with the dermal (glossy) side directly in contact with the wound surface
Using a saline-moistened cotton tip applicator, smooth Apligraf onto the wound bed so there are no pockets or wrinkled edges
 2A-2B
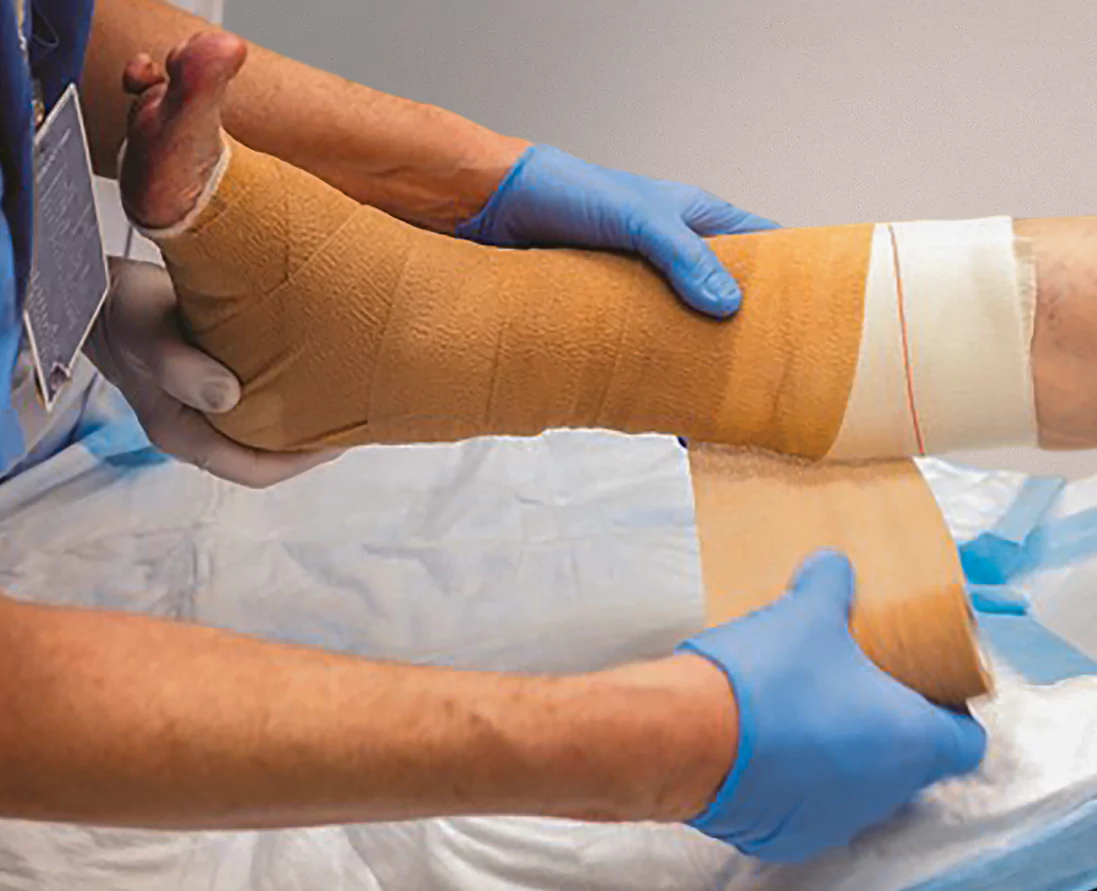
2A-2BStep 3: Applying dressing
Cover Apligraf using a non-adhesive primary dressing
Anchor Apligraf using clinician’s choice of fixation
Apply a secondary, non-occlusive dressing to create a bolster
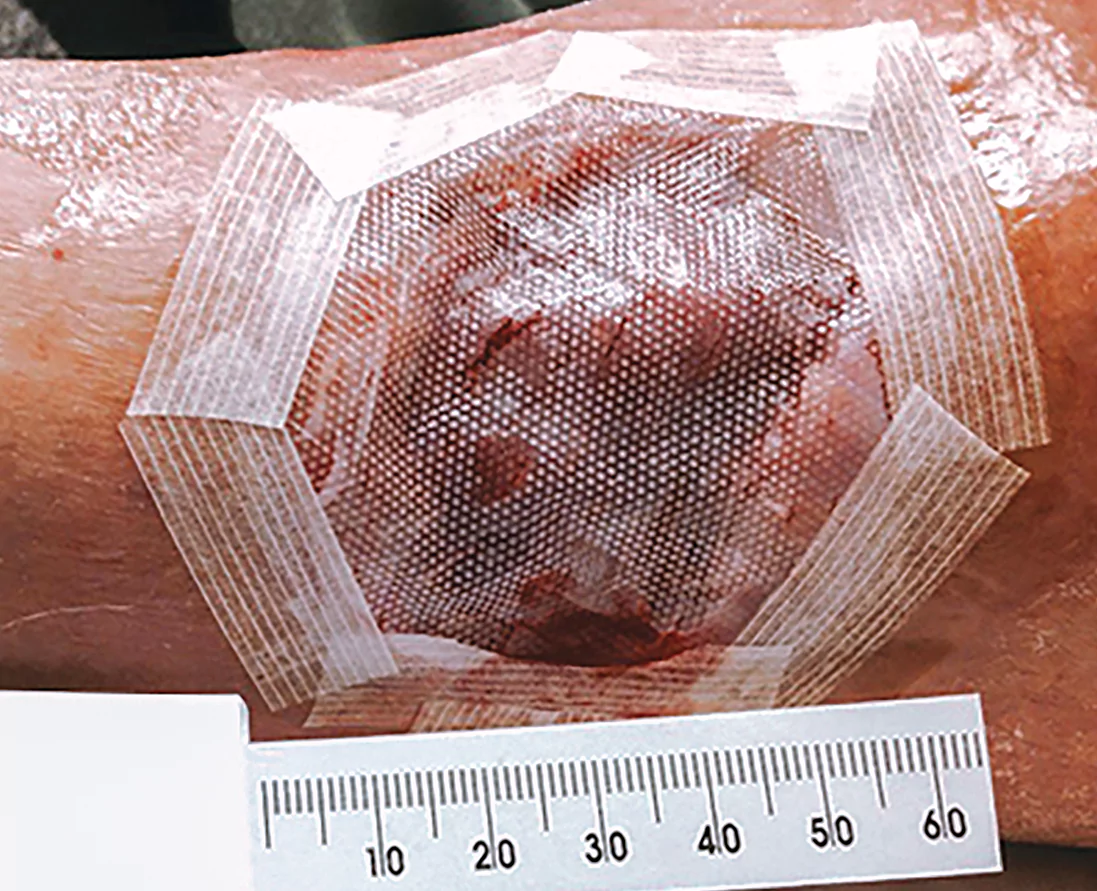 3A-3B
3A-3BCompress/offload and follow-up
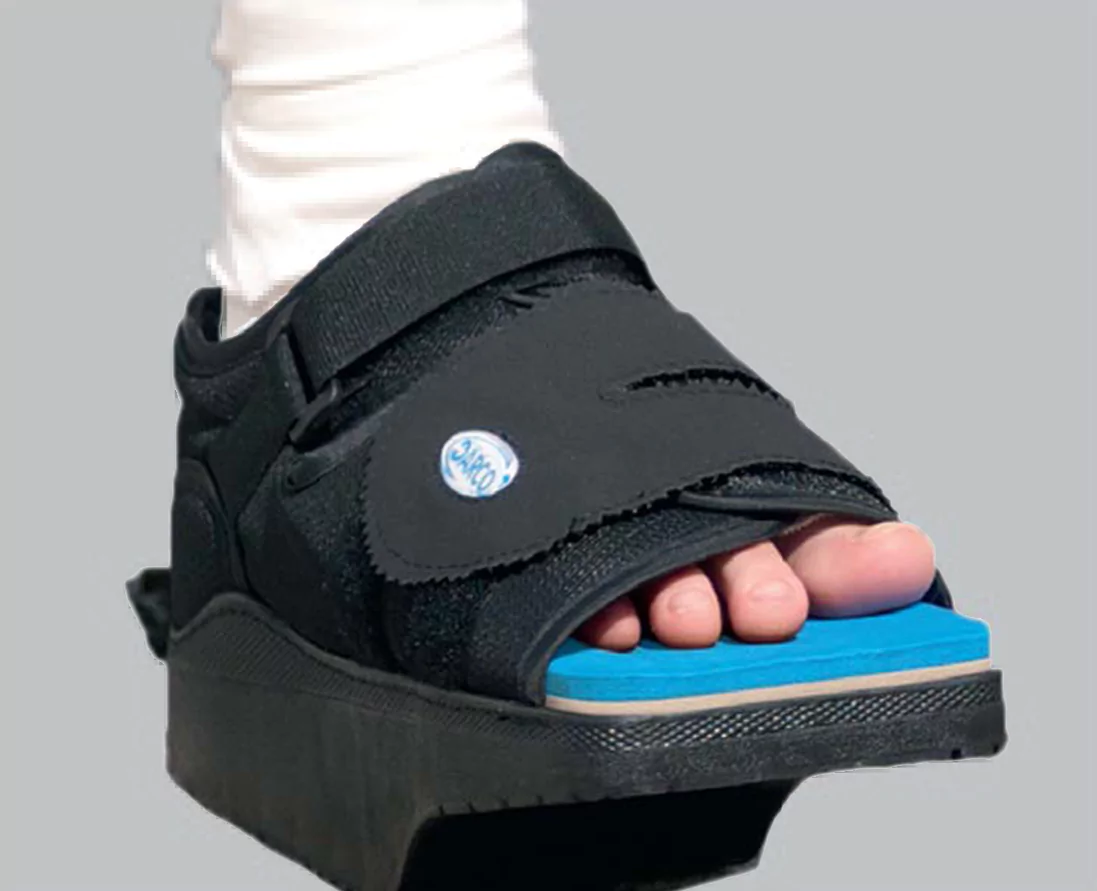
- Apligraf should always be used with appropriate compression or offloading as well as good wound-care practices
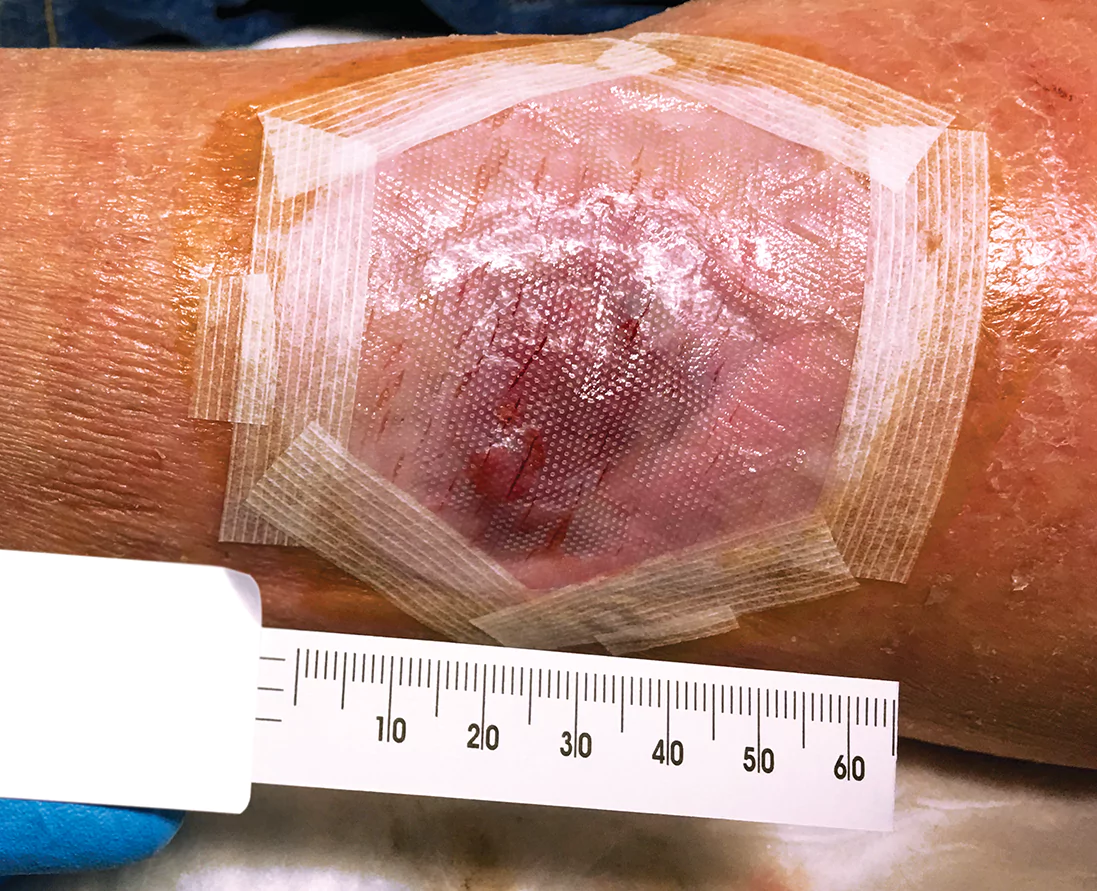
- Reassess the wound weekly, and reapply Apligraf weekly as long as the wound continues to respond*
- Debride the wound while not disrupting healing tissue
- Clean the wound with a noncytotoxic solution
Downloadable resources
| Product Number | Apligraf Product Description | Total Size (cm2) | Billable Units | HCPCS Code | UDI Number |
|---|---|---|---|---|---|
| APLIGRAF-COM | Apligraf (living, bi-layered skin substitute) | 44 | 44 | Q4101 | 00618474000008 |
Proven results in clinical trials and real-world settings
Examine the clinical and real-world evidence for Apligraf, or contact an Organogenesis Tissue Regeneration Specialist today to get more information about how Apligraf can help your VLU and DFU patients.
Contact usPlease refer to the Apligraf Package Insert for complete prescribing information.
REFERENCES:
- Apligraf [package insert]. Canton, MA: Organogenesis Inc; 2017.
- Data on file. Organogenesis Inc.
- Veves A, et al. Diabetes Care. 2001;24(2):290-295.